Weliky Lab - Research
The Weliky group is interested in mechanistic and structural understanding of biochemical systems. We are primarily experimentalists and typically prepare our own samples and do our own measurements and data analysis.
Protein Expression and Purification and Biophysical Characterization
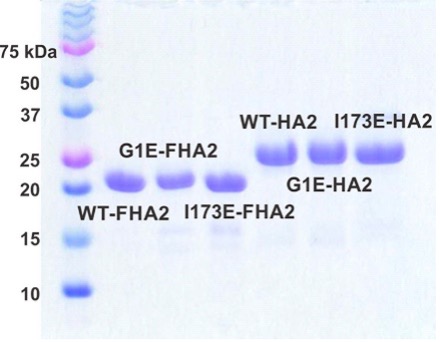
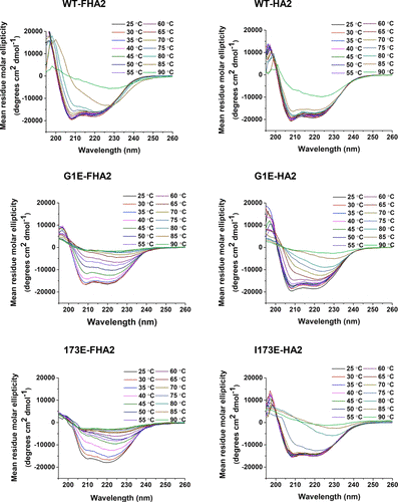
On the sample preparation side, we do protein expression and purification as well as characterization using methods like SDS-PAGE, mass spectrometry, circular dichroism spectroscopy, and size-exclusion chromatography. For protein purification, we are typically developing the more “old-school” differential solubility approaches while also using the more “modern” chromatographic approaches.
Click the thumbnails to see more:
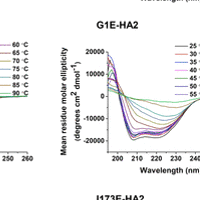
NMR Methods and Approaches
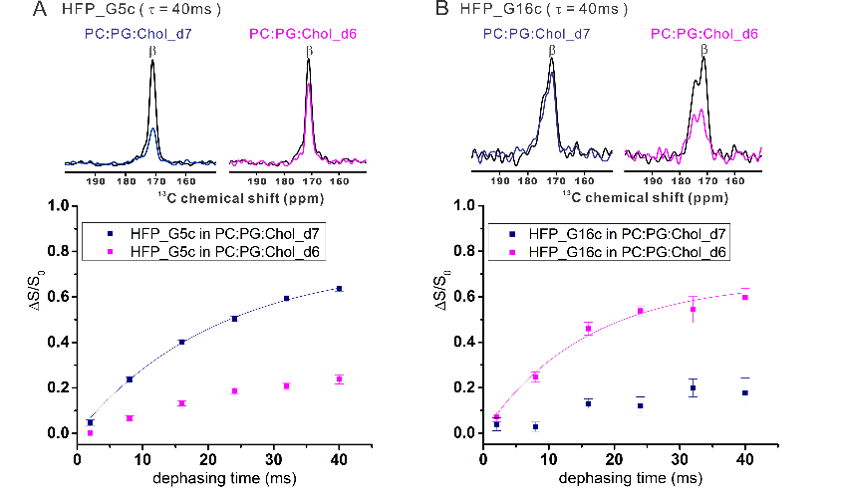
We do significant nuclear magnetic resonance (NMR) spectroscopy. Some NMR projects have the goal of structure determination with near-atomic resolution. Other projects using NMR to address questions like protein location in a membrane or the probability of lipid chain protrusion out of a membrane.
Click the thumbnails to see more:
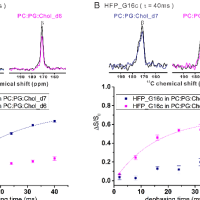
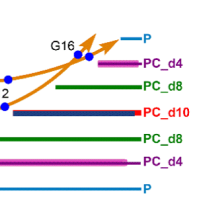
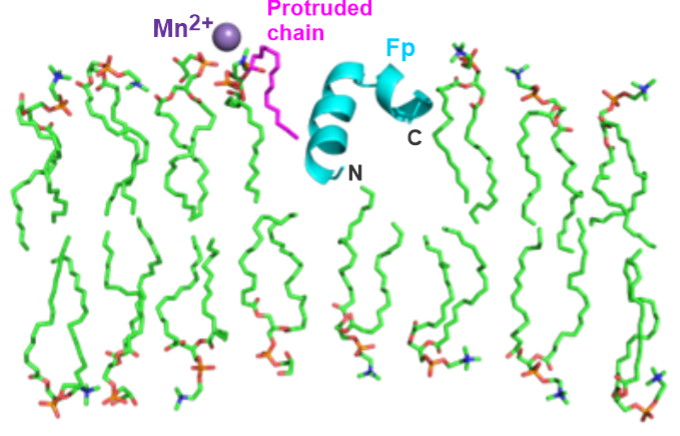
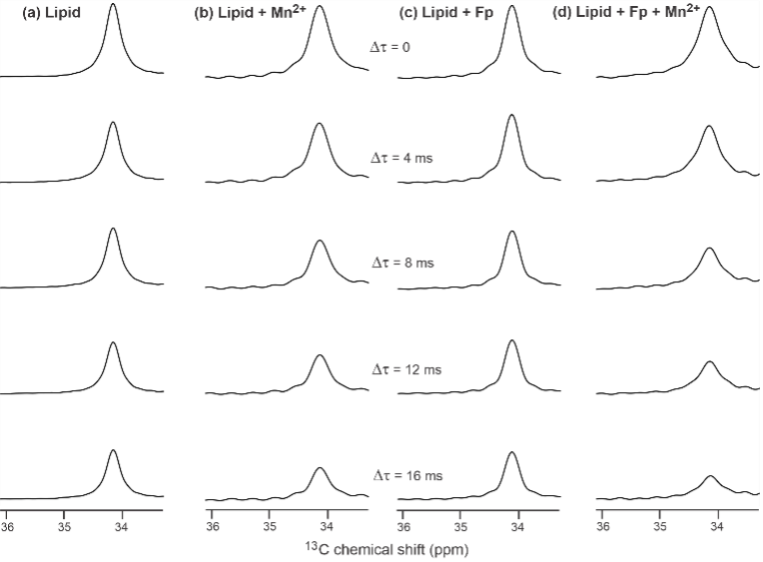
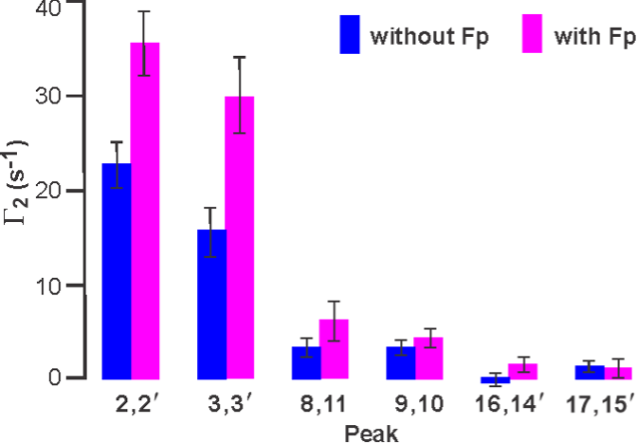
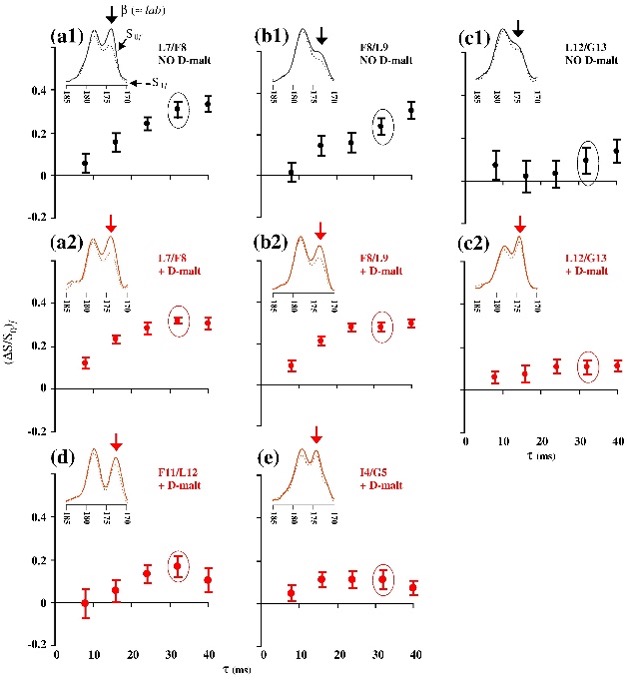
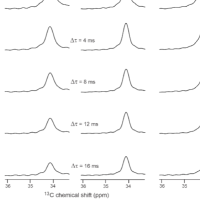
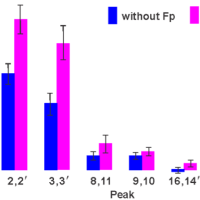
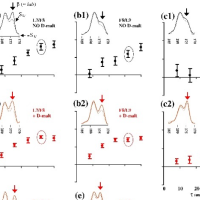
Protein-induced Changes in Lipid Structure by NMR Paramagnetic Relaxation Enhancement
Click the thumbnails to see more:
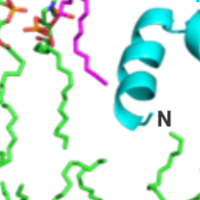
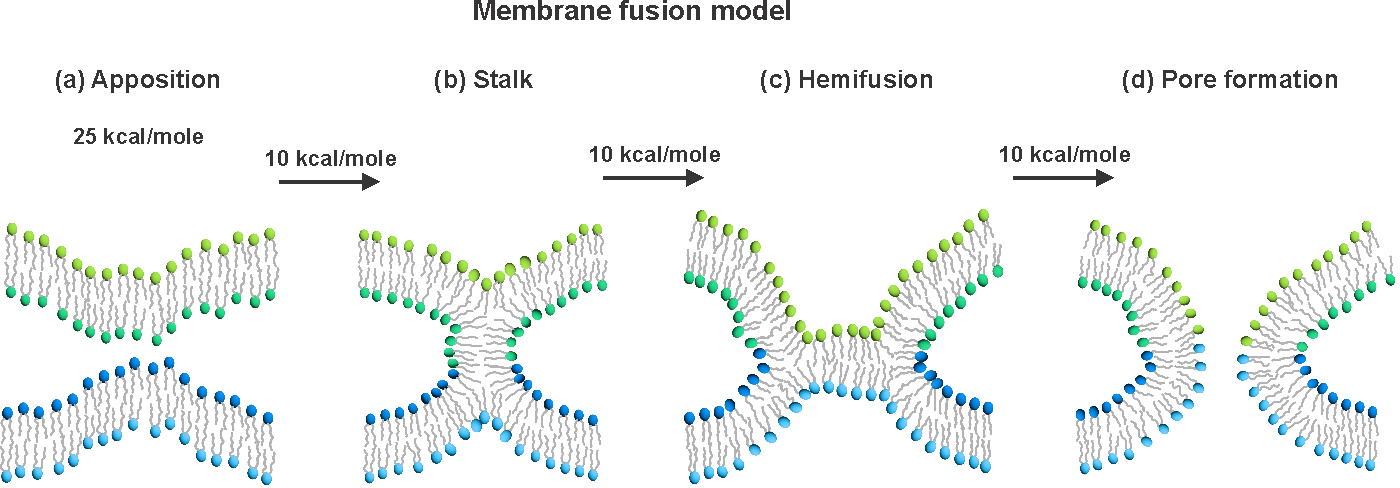
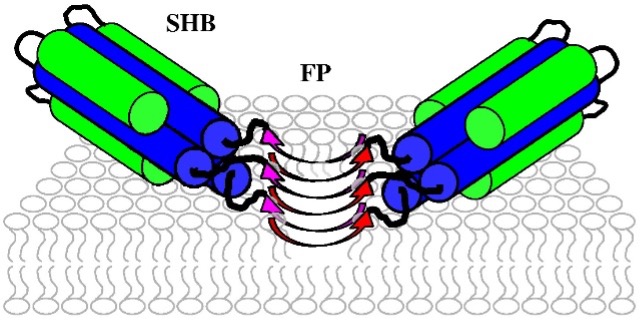
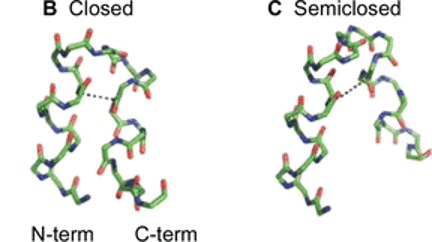
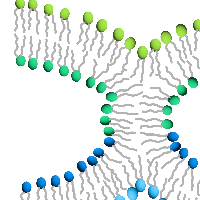
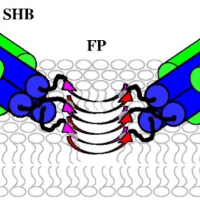
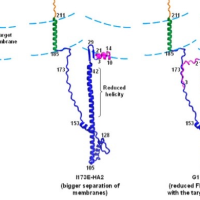
Enveloped virus spike proteins
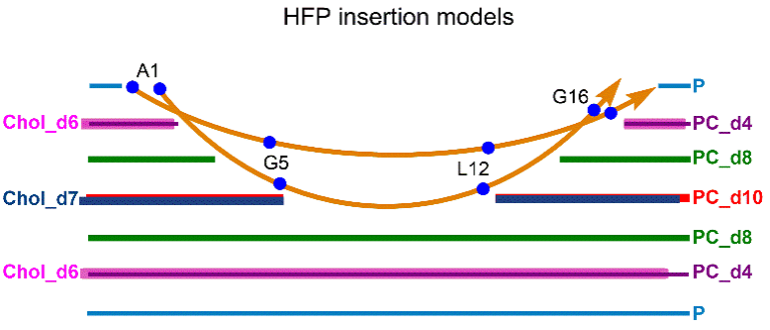
Many viruses important in disease are enveloped by a membrane obtained during budding from an infected host cell. Infection of a new cell requires joining (“fusing”) the viral and target membranes to enable viral replication in the cell. Fusion has substantial barriers that are reduced by one of the subunits of the viral protein spikes. Our work is focused on determining the mechanisms by which these barriers are reduced by the spike proteins.
Click the thumbnails to see more:
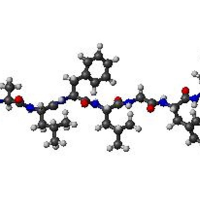
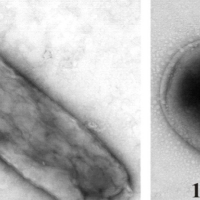
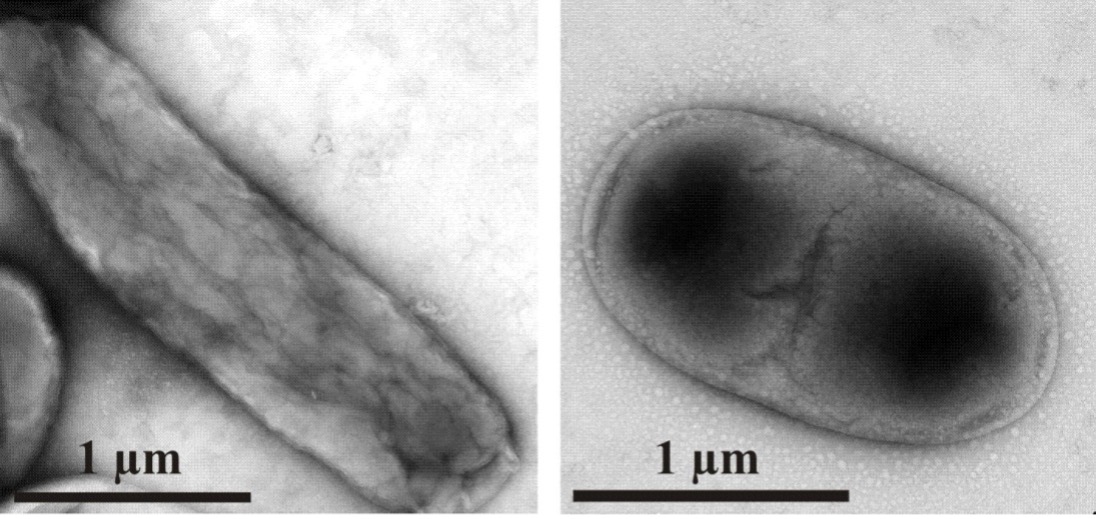
Proteins in Bacterial Inclusion Bodies
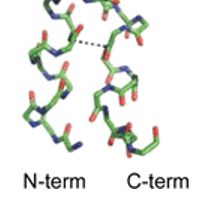
With genetic manipulation, bacteria can be induced to synthesize any protein sequence. Typically most of the synthesized protein is aggregated as an “inclusion body”, a solid object in the bacterial cytoplasm. We are doing NMR and other measurements to understand the extent to which the recombinant protein adopts native structure in an inclusion body and the intermolecular interactions that underlie the aggregation.
Click the thumbnail to see more: